Charting the Future of Immunology & Immunotherapy with Michelson Philanthropies & Science Prize Winners
On April 21, 2022, Keystone Symposia will be hosting a free ePanel event to celebrate the inaugural winners of the Michelson Philanthropies & Science Prize for Immunology, an international prize that focuses on transformative research in human immunology, with trans-disease applications to accelerate vaccine and immunotherapeutic discovery.

The event will showcase innovative research in immunology and immunotherapy that have provided insights into viral pathology, treatments and vaccine responses, in the context of COVID-19 and beyond. Altogether, these scientists are providing the discoveries and tools to improve our response to the current pandemic, and preparedness for the next.
Their research and its importance to society will be put into context with welcoming remarks by scientific and political leaders and those who selected the award recipients. Prize winners and finalists will then present their work and answer questions from the global virtual audience.
-
Dr. Gary Michelson, Michelson Philanthropies founder and co-chair
-
Bill Moran, Publisher of AAAS/Science
-
Seth Scanlon, Editor of AAAS/Science
-
United States Senator Alex Padilla of California
Learn more about the award winners and their research below!
Meet the Michelson Philanthropies & Science Prize Winners
The Michelson Philanthropies & Science Prize for Immunology is awarded annually as one $30,000 grand prize, and two $10,000 finalist awards. The prize recognizes transformative research in human immunology, with trans-disease applications to accelerate vaccine and immunotherapeutic discovery.
The winners of the prize were selected through a global competition where their essays, explaining how their novel approaches have advanced immunotherapy research and how it will have a lasting impact, were reviewed by a distinguished committee of scientists chaired by Science editors.
Paul Bastard – WINNER
Why Do People Die from COVID-19?
Laboratory of Human Genetics of Infectious Diseases at Necker Hospital for Sick Children, Imagine Institute, INSERM & University of Paris, The Rockefeller University, New York

Paul Bastard, MD, PhD, is currently working as a chief resident in the Department of Pediatrics at the Necker Hospital for Sick Children (AP-HP, Paris, France), while also doing research in the Necker branch of the laboratory of Jean-Laurent Casanova, located at the Imagine Institute (University of Paris and INSERM) and the Rockefeller University (New York, USA). His research focuses on the genetic and immunological determinants of severe viral diseases, including the causes and consequences of autoantibodies against type I interferons.
RESEARCH ABSTRACT:
Autoantibodies neutralizing type I interferons increase with age
Although millions of people have suffered from life-threatening COVID-19, the course of infection has been benign in many more individuals. Aging is the major risk factor for life-threatening disease, the risk doubling every five years from childhood onward. What are the molecular and cellular mechanisms underlying such vast and age-dependent clinical heterogeneity? The COVID Human Genetic Effort recruited patients with all clinical outcomes, ranging from silent infection to lethal disease. We searched for both inborn errors of immunity (IEI) and auto-immune phenocopies of these IEIs. We found IEIs affecting type I IFN and auto-Abs neutralizing type I IFNs as being causative of life-threatening COVID-19 pneumonia in about 20% of cases. These auto-Abs pre-exist infection and increase sharply in those over 65 years old.

Scott B. Biering – FINALIST
One Antibody to Treat Them All
Division of Infectious Diseases & Vaccinology, School of Public Health, University of California, Berkeley

Scott Biering received undergraduate degrees from the University of California, Los Angeles and a PhD in Microbiology from the University of Chicago. He is currently a postdoctoral scholar at the University of California, Berkeley in the laboratory of Dr. Eva Harris. His present research investigates the role of viral proteins like flavivirus nonstructural protein 1 (NS1) and SARS-CoV-2 spike (S) in inducing viral pathogenesis and promoting viral dissemination.
RESEARCH ABSTRACT:
Conserved flavivirus protein holds potential as target for versatile vaccines & therapies
Flaviviruses are a group of medically important viral pathogens which cause diverse disease pathologies and significant global disease burden. A contributing factor to flavivirus pathogenesis is the conserved non-structural protein 1 (NS1), which triggers vascular leak through interactions with endothelial cells. While NS1-specific antibodies have been shown to be protective against flavivirus infection, the mechanism by which they protect is unknown. To determine how anti-NS1 antibodies protect against flavivirus infection and how NS1 triggers pathogenesis, we solved a crystal structure of a protective and cross-reactive monoclonal antibody 2B7 in complex with dengue virus NS1. Our structure revealed that 2B7 blocked two distinct domains of NS1 from interacting with endothelial cells. Further, we found that 2B7 bound strongly to diverse flavivirus NS1 proteins, providing protection from dengue and Zika virus infections. Our structural and mechanistic investigation of anti-NS1 protection revealed critical domains for NS1 function and serves as a proof-of-concept for single antibody pan-flavivirus protection.
Lisa Wagar – FINALIST
Small Centers of Defense
Institute for Immunology, University of California, Irvine
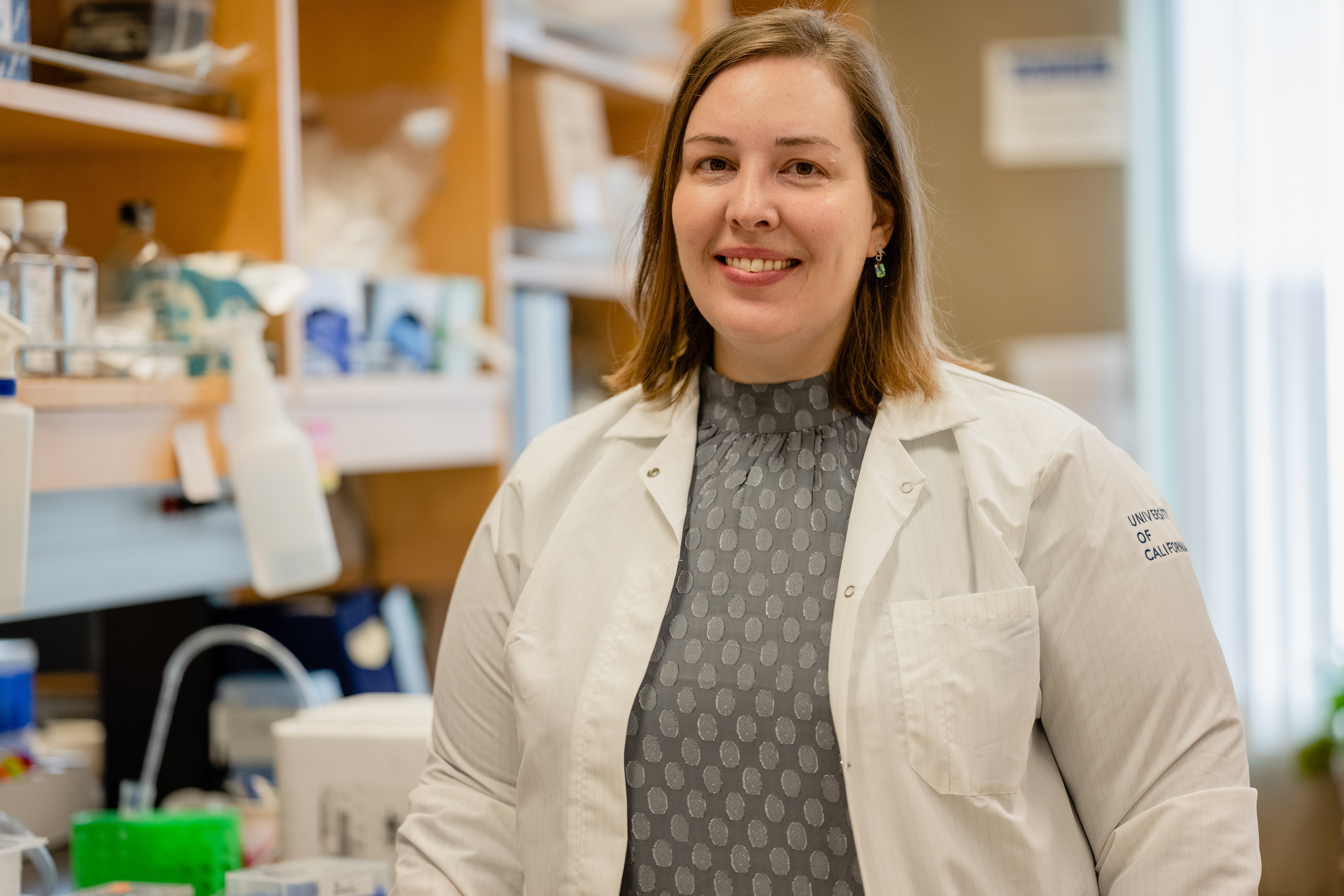
Lisa Wagar received a BSc from the University of Ontario Institute of Technology and a PhD from the University of Toronto. After completing her postdoctoral fellowship at Stanford University, Dr. Wagar started her lab in 2020 in the Department of Physiology and Biophysics at the University of California, Irvine where she is currently an Assistant Professor. Her research focuses on translational human immunology and the use of organoids to understand the complex interactions that occur between immune cells upon vaccination and infection in humans.
RESEARCH ABSTRACT:
Deciphering immune responses to viruses and vaccines using human tonsil organoids
Recent advances in organoid technologies have enabled a deeper understanding of the complex cell-cell interactions occurring within human tissues. Adaptive immune responses to vaccines, infectious diseases, and other antigens rely on many cell types dynamically organizing within lymph nodes and other lymphoid tissues. We recently developed an immune organoid platform derived from primary tonsil tissues, a lymph node-like secondary lymphoid tissue, to support in vitro analysis of human adaptive immunity. Tonsil organoids produced a multifaceted immune response to influenza and other vaccine-relevant antigens, as evidenced by antigen-specific B cell and T cell activation, differentiation, and function. The organoids accurately reflected in vivo aspects of interindividual variation, such as age-related differences in the magnitude and quality of the antibody response. In the future, it is our hope that the knowledge gained from in vitro studies of human adaptive immunity will lead to improved vaccination and immunotherapeutic strategies.
Register Now to See These Award Winners Present Their Work!
This ePanel event is organized in collaboration with:
Related news
Michelson Philanthropies & Science Prize for Immunology ePanel
On April 11, we celebrated the 2022 recipients of the Michelson Philanthropies & Science Prize for...
Michelson Philanthropies & Science Prize for Immunology ePanel, September 10, 2024
On September 10, 2024, Keystone Symposia will host a free ePanel event featuring the recipients of...
Michelson Prizes- Next-Generation Grants: Advancing Immunology and Vaccine Innovation ePanel
On March 24, 2023, Keystone Symposia hosted a live ePanel event featuring the recipients of the ...






